Mole Calculations Explained – Formula mass and mole calculations
Want to know how to calculate moles? Need to convert grams to moles, or moles to grams? Then welcome!
You are watching: Mole Calculations Explained – Formula mass and mole calculations
A mole, in chemistry, is how chemists define an amount of substance, useful when dealing with many different molecules reacting at once, for example any chemical reaction. The official International System of Units definition is that a mole is, “the amount of a chemical substance that contains exactly 6.02214076×1023 (Avogadro’s constant) atoms, molecules, ions or electrons (constitutive particles), as of 20th May 2019. Prior to that, a mole was defined as the number of atoms in 12 grams of carbon-12 (an isotope of carbon).” That was a mouthful!
Why do Chemists use moles and why do you need to calculate it? It provides a useful metric when dealing with reactions, let’s look at any example:
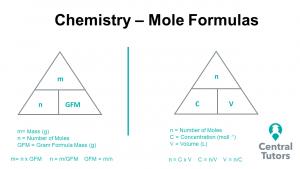
“Let’s say you want to neutralise 10 g of hydrochloric acid (HCl in water) with some sodium hydroxide (NaOH). Now, you want the resulting solution to be perfectly neutral, so you don’t want to add too much or to little NaOH, making it too basic or acidic respectively. It is therefore useful to find out exactly how many molecules of HCl are in the solution. This is where moles come in handy. To know how to calculate moles, the equation is:
mole = mass / molecular weight”
See more : The Rise of MetaZooMee: Revolutionizing Virtual Interactions
Let’s do any example on how we can convert moles to grams, or grams to moles.
“We know we have 10 g of HCl, and it has a molecular weight of 36.5 g / mol. Lets plug these numbers into the above equation:
mole = 10 / 36.5 = 0.27 moles = 1.626×10^23 molecules of HCl
We can work out the number of molecules by timesing the moles by Avogadro’s constant above. Now we know the amount of molecules of HCl we have, and, since the reaction is 1:1, we need the exact same number of molecules of NaOH to neutralise it. As we need to find the mass of NaOH to add, lets quickly rearrange the equation:
mole = molecular weight / mass (mulitply both sides by mass)
mole * mass = molecular weight (divide both sides by mole)
mass = molecular weight / mole
See more : Life Insurance Quotes for Seniors in USA: Your Guide to Affordable Coverage in Your Golden Years
As 1.626×1023 molecules of NaOH is also equal to 0.27 moles, and we know that the molecular weight of NaOH is 40, we can use these numbers to get:
mass = 40 / 0.27 = 10.8 g
So we now know we need 10.8 g of NaOH to exactly neutralise our amount of hydrochloric acid.”
1 mole is the amount of a substance that contains exactly 6.022×1023 of something. While this something could be anything, because it is such a large number it is usually reserved for atoms, molecules, electrons, and ions.
Much of the information for this blog post was found on https://www.omnicalculator.com/chemistry/mole
Central tutors provide classes in the SQA Higher Chemistry. If you are looking for a National 5 chemistry tutor or Higher chemistry tutor we’d love to hear from you. For more information please visit, www.centraltutors.co.uk or contact us on info@centraltutors.com
Or follow the link to contact us and book an introductory slot for chemistry tuition
Source: https://en.congthucvatly.com
Category: Blog
This post was last modified on Tháng Mười 5, 2023 6:20 chiều
If you're a 60-year-old man considering a $500,000 life insurance policy, you're likely wondering about…
Choosing the best life insurance provider is a crucial decision, especially in Canada, where numerous…
Navigating the U.S. financial landscape as a foreigner can be complex, and life insurance is…
American Family Insurance is a well-established provider known for its comprehensive range of insurance products,…
While banks primarily offer financial services like checking and savings accounts, loans, and credit cards,…
Chase, a well-known financial institution, offers a wide range of financial products and services. But…